Top Pharma Manufacturing Companies in India
Our company comes under the Top Third Party Manufacturing Pharma Companies in India. With the help of our large-scale production unit, we are capable to deliver the variety of drug range to our associates. Being one of the most reputed third party pharma manufacturers, we have the capacity to deliver all type of drug ranges such as a pellet, tablet, capsule, Herbal Nutraceuticals, protein powder, Gym supplements, Capsules, Tablets and pharma sachets. Being a leading 3rd Party Manufacturing Pharma Company, we make use of best quality raw material and cutting-edge tools.
We are linked with the best distribution channels in India, who help us to make the on-time delivery of all the manufactured product. Our domain lies in the list of Top Pharma Manufacturing Companies in India. Along with the quality of drugs, we also ensure the quality packaging of all the produced drugs. Being the top Third Party Pharma Manufacturers in India currently, we are providing our service to around 500+ pharma companies from all around the nation.
Mentioned Below are some of the pharma manufacturing services we provide:
About Third Party Manufacturing Pharma & Contract Manufacturing Facility
Product Approvals – We Have Both Beta Lactam ( Complete Antibiotic Range ) & Non Beta Lactam Manufacturing Approvals . We Provide All Services Regarding Manufacturing Like , Private Label Manufacturing , Custom Formulation Manufacturing , ODM Manufacturer.
Product Sections Available In Manufacturing Plants/Unit With Production Capacity:
| Tablet | 2.46 Billion Units Tablets(PerAnnum) |
| Injections /Respules | Billion Units Injections (Per Annum) |
| Liquid / Syrup | 7.26 million liters Liquid (Per Annum) |
| Capsules | 186.2 Million Units Capsules (PerAnnum) |
| Drops | Millions Units Drops (Per Annum) |
| Inhalers | Millions Units Inhalers (Per Annum) |
| Sachet | Millions Units Sachet (Per Annum) |
| Powder | Millions Units Powder (Per Annum) |
| Soft Gel | 136.2 Million Units Capsules (Per Annum) |
| Gel | Millions Units Gel (Per Annum) |
| Spray | Millions Units Spray (Per Annum) |
| Pallets | Millions Units Pallets (Per Annum) |
| Dry Injection | Millions Units Dry Injection (Per Annum) |
| IV Fluid | Millions Units IV Fluid (Per Annum) |
| Nasal Spray | Millions Units Nasal Spray (Per Annum) |
Different Range For Third Party Manufacturing

Derma & Cosmetic

General

Dental

ENT

Gynae

Nephrology

Oncology
Urology

Ortho

Critical Care
Cardiac

Pediatric
Diabetic

Food Supplements & Nutraceutical

Ophthalmic

OTC
Our Certification
Our Product Manufactured At International Standard, Plant Have All Certification Required To Match International Standard
Documents Required for 3rd Party Manufacturing
Profile:
(Brief Profile with Copy of PAN Card of the company and a Copy of Memorandum & Articles of Association in case of Pvt. Ltd or limited Company. Partnership deed/ Affidavit for proprietary in case of Partnership firm or Proprietorship firm.)
Name, Address & Telephones with copy of Aadhar Card and Pan Cards.
(Of all Directors, Partners or Proprietor both official and residential.)
Copy of Resolution for Authorized Signatory to Deal
(For limited, private limited and partnership companies)
Drug Licenses:
(Attested copy of Drugs Licenses to be provided)
GST Registration Certificates:
(Attested copy of Sales tax Registration Certificate to be provided)
Agreement for Manufacturing:
(Specimen Copy Will Provide)
Certificate for Non-Resemblance:
(Specimen Copy Will Provide)
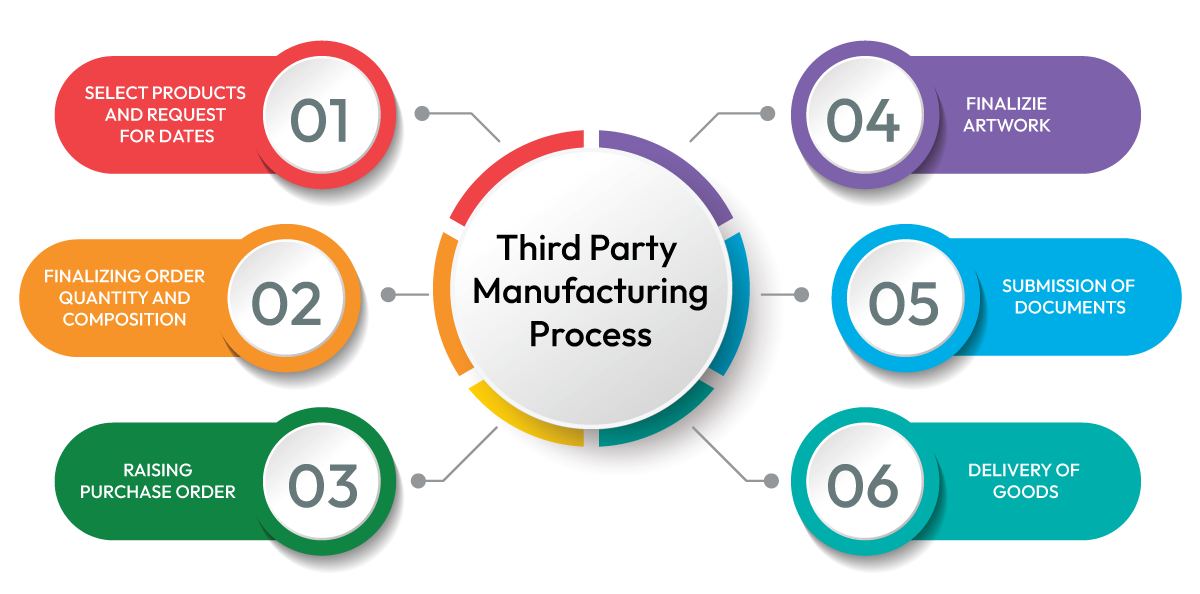
PROCESS OF THIRD PARTY CONTRACT MANUFACTURING
Third Party Manufacturing business in the pharma industry is growing at very fast pace globally and is expected to increase at a very fast rate over the next few years. Every manufacturing business company is using quite similar technologies and equipment.
The third party manufacturing process consists of multiple steps, and for each step there are process parameters with some control limits.
- Finalizing order quantity and composition
- Raising quotation
- Required documents
- Packaging material
- Product manufacturing
- Product delivery
Expected Investment Required for Allopathic Or
Ayurvedic Third Party Products Manufacturing Process
What Does Inventory Require to Get Manufactured Pharma / Ayurvedic product at Third Party Basis?
Inventory may vary according to your product selection, dosage form, packing material requirement etc. Your inventory also may vary according to batch size of product. Less expensive product will require less investment but their batch size may large in quantity. Likewise costly products require more inventory but their batch size may be small in quantity. Some manufacturer take small batch for all products, hence one have to invest less. There is not a fixed amount of investment for getting manufactured your product at third party basis. Roughly you can calculate your investment according to below given information’s
Tablet/Capsule
Batch Size: 25000 tab/cap
Pack: 10*10 Blister/alu-alu
- Packing material require: 250 Box and roughly 2-3 kg foil. But printer will not print such small quantity. You have to print minimum 500 or 1000 boxes and 5 kg of foil. One time 1100/- cylinder charges for one color and 2200/- for two color foil cylinder charges, you have to pay to printer.
- Estimated cost will be calculated as per 1000 box printing material:
- Box= 1000*1.5=1500
- Foil=5*450=2250
- Cylinder charges: 1500
- Total packing material inventory: 5250/-
- Total number of box to be manufactured is 250*90=22500
- Packing material inventory= 5250
- Total cost= 27750/-
- Now calculate the costing for first batch: 27750/250=111/- per box.
- Now calculate the costing for next batch: 22500/250=90/- For next 2 batch, you may not require new box printing but you may require some foil. Here we are calculating costing for first two batches only.
- Average costing= 111+90= 201/2= 100.5/- per box.
Ointments/Creams/Gel
Batch Size: 1000 pc
Pack: 20 gm with outer cartoon
- Packing material required is 1000 pc
- Box: 2000 pc*1/-= 2000/-
- Lami tube: 10000 pc*2/-=20000/-
- Cylinder charges for one color is 1500/- and for two color 2500/-(one time investment)
- Total Packing material: 23500/-
- Manufacturing cost: 6.5*1000= 6500/-
- Packing material inventory: 23500/-
- Now calculate the costing for first batch: 30000/1000=30/- Per pc
- Now calculate the costing for next batch: 6500/1000=6.5/-
- After next 10 batches, you don’t need to invest in Lami tube. But after 2000 pc, you will need boxes for this and you can add 1/- per pc more costing.
- Now costing for next 10 batches costing will be 7.5/- per pc
- Average costing: 30+6.5+7.5+7.5+7.5+7.5+7.5+7.5+7.5+7.5=96.5/10=9.65/- per pc.
Liquid/Syrup
Batch Size: 1000 pc
Pack: 60 ml. with outer cartoon
- Box: 2000 pc*0.75/-= 1500/-
- Label: 5000 pc*0.10/-=500/-
- Total Packing material: 2000/-
- Manufacturing cost: 6.5*1000= 6500/-
- Packing material inventory: 2000/-
- Now calculate the costing for first batch: 8500/1000=8.5/- Per pc
- Now calculate the costing for next batch: 6500/1000=6.5/-
- Average costing: 8.5+6.5=15/2=7.5/- per pc
Injections
Batch Size: 2000 pc
Pack: 1 ml with outer cartoon
- Packing material required is 5000 pc
- Box: 5000 pc*0.40/-= 2000/-
- Label: 5000 pc*0.05/-=250/-
- Total Packing material: 2250/-
- Manufacturing cost: 3*2000= 6000/-
- Packing material inventory: 2500/-
- Now calculate the costing for first batch: 8500/2000=4.25/- Per pc
- Now calculate the costing for next batch: 6000/2000=3/-
- Average costing: 4.25+3=7.25/2=3.63/- per pc
Above costing is for example purpose only. It is not the actual cost sheet of any product. According to above basis, you can calculate, how much you have to invest initially to start you own pharma marketing company.
Product manufactured from Leading Pharma Manufacturing Units





Frequently Asked Questions
-
What is Pharma CMO (contract manufacturing organization) ?
A contract manufacturing organization (CMO) is an organization that serves the pharmaceutical industry and provides clients with comprehensive services, from drug development to manufacturing. Outsourcing to a CMO allows the pharmaceutical clients to expand their technical resources, without increased overheads.
-
What is third-party manufacturing in Pharma?
Generally, third-party manufacturing is a process of manufacturing medicines and products from other parties. In this process, signing an agreement is done between the company and the manufacturing company, and the manufacturing company manufactures the products as per the contract.
-
What is the difference between contract manufacturing and third party manufacturing?
In third party manufacturing, there is no time compulsion to deliver the goods whereas contract manufacturing has delivery time compulsion. Mostly small companies work at the basis of third party manufacturing whereas medium size and big companies work at contract manufacturing basis
-
What is the procedure for packing material Procurement, Inventory, type in Pharmaceutical/Ayurvedic Manufacturing Company?
What is packing material for pharmaceutical/Ayurvedic products?
Packing material is outer covering in which dosage form is packed. It may in form of paper or plastic or rubber or tin or in other form. Packing material includes Outer cartoon, mono cartoon (if applicable), foil, label, lami tube etc. Generally vial, ampoule, protein powder tin, pet bottle don’t be considered in packing material but these are also the part of packing material. It is considered to be part of product. Manufacturer will give you rate including bottle, vial etc even if you will ask for rates without packing material. In pharmaceutical industry, there is norm that must be printed at packing material.
Types of Packing Material:
- Box
- Foil
- Label
- Lami Tube
- Inner/mono ( if applicable)
Costing of Packing Material:
Cost of Packing Material may vary according to Size and Quantity Tablets/Capsules ( Per Lac ) One time cylinder charges(Single color) foil 1500 One time cylinder charges(Two color) foil 3000 Foil= 5kg* 450 2250 Box Charges(Outer Cartoon) = 1000 box * 2 2000 Mono cartoon (If applicable) = 10000*1 10000 Total Approximate costing for Packing material Single color with outer 5750 Double color foil with outer 7250 Single color with mono cartoon 13750 Double color foil with mono cartoon 15250 Liquids/Syrups/Dry Syrup (Per 1000 pc) Lable= 1000*.10 100 Outer = 1000*.75 750 Total Approximate packing cost for 1000 pc 850 Ointment/Gel/Creams One time cylinder charges(Single color) foil 1500 One time cylinder charges(Two color) foil 3000 Lami Tube= 1000*2 2000 Box= 1000*1 1000 Total Approximate packing cost 7500 Injections/Eye-Ear Drops(Per 1000 pc) One time cylinder charges(Single color) foil(If applicable) 1500 One time cylinder charges(Two color) foil (If applicable) 3000 Foil = 5 kg *450 2250 Label= 1000*.10 100 Outer = 1000*.75 750 Inner/mono cartoon (if applicable) Total Packing Material cost Without Blister pack 850 With Blister Pack 7600 What are these norms? Is it necessary to follow these norms?
Timely, Government and health department change the norms. In drug and cosmetic act, there are mentioned different schedules. Packing material of pharmaceutical products should compile with rule under these schedules. For products that can be sold under only doctor’s prescription, should mention warning and Rx should also be placed above salt name. Warning, caution, dosage etc should be mentioned according to these schedules. For example, newly implement Schedule H1 is must for antibiotics that come under this schedule. For Ayurvedic Products norms are different from pharma products.
Who is responsible for correction of these norms? Marketing company or Manufacturer!
Correction and implementation, checking of matter is full responsibility of manufacturer. If product fails to comply with these norms, drug department may issue notice to manufacturer and marketed by both. But manufacturer is considered to be the main party for violating the rule.
What is the role of marketing companies in packing material procurement process?
There is two way to procure packing material in third party manufacturing. One you can directly involve in printing of packing material or you can let it to manufacturer to procure it for yourself. Likewise manufacturer will give you rates at third party basis with packing material or without packing material. Cost will be nearly same in both cases.
What is the procedure for the printing of packing material for pharmaceutical/Ayurvedic products?
Before starting manufacturing process one need to print the packing material for the same. Packing and design of the products make first impression at mind of customers. In past when these modern equipment were not available, companies used to write at plain paper to describe the product. We will recommend you to procure packing material yourself but if you don’t have time and staff to look after printing process, you should let it to the manufacturer.
Procedure of Packing Material printing:
- Select printing material supplier and manufacturer.
- Prepare design for the packing material. Finalize best design.
- Select paper for packing material for cartoons and labels.
- Send it for correction and approval to the manufacturer for technical corrections.
- After correction and approval, send it for printing.
If anyone doesn’t want to involve in printing of packing material then what is to do?
Manufacturers provide both options, with packing material or without packing material. You can ask manufacturer to procure packing material. You just have to finalize design sent by manufacturer or manufacturer’s printer. You have to check for brand name, composition, marketed by address or other particulars, you want to change.
If anyone want to pack his products in special packing i.e. in special pet bottles, tin, vial, ampoules or in special dye!
Manufacturer will make your product with respect to your specifications. But that depends upon availability of these materials. If manufacturer is not able to procure these materials, you can also send these materials to manufacturer by yourself.
-
How to ensure quality of products in third/contract manufacturing?
Query:
We were planing to start pharmaceutical marketing company, read your posts. we found it very helpful. Still we have one confusion; if drug product manufactured by third party shows some adverse reactions then in that case DCGI take action against whom marketing or manufacturing company? and what will be the penalty for accused?
Response:
Manufacturer will manufacture only DCGI approved molecules. These molecules and combinations are already tested toward adverse reactions. In some cases if products show any adverse reactions or drug department found product of substandard quality then manufacturer will be responsible for it. Marketing company has to show proof of sale and purchase in that case and satisfy the drug department regarding marketing company don’t have any knowledge about substandard quality of product.
But its also responsibility of marketing company to take quality report along with product or test each batch at independent laboratory for quality and efficacy of product.
DCGI can make accused manufacturing company or in some cases it make accuse all distribution channel along with marketing company. Penalty could be only fine or imprisonment or both depend at severity of matter.
Query:
I’d like to ask you one more question. Though manufacturing company has already got the approval for drug product from DCGI. But, what if they produce drug products which are not having API strength specified or they provide products with 100% assay value but product not remain stable during shelf life and undergo degradation, hence reduction in assay value. As a marketing company we are planning for contract manufacturing, so in that case how can we ensure quality of products? Can you please reply on this? I would be really grateful if you could answer.
Response:
In case of product that are not produced with quality standard by manufacturer or not having API strength as specified. You have two option:
First ask COA (Certificate of Analysis) along with Batch you received from manufacturer.
Second send few sample to independent laboratories for testing. Many laboratories provide facility for testing products and will give you assay report of batch you received from manufacturer.
-
What is the Minimum Order Quantity (MOQ) for third party manufacturing?
It Depends Upon Company As Well As Product Section , Its Varies Company To Company , Party Can Negotiate With Manufacturing Company .
-
Why do third party/Contract Manufacturer require Trade Name/ Brand Name Affidavit?
Query:
Requesting your help regarding drug brand name registration I run a proprietorship company in Bangalore pcd business already but want to enter contract manufacturing. Companies are demanding brand Name Affidavits. I just want to clarify that
Response:
Companies demand brand name Affidavit for avoiding any future problem. In Pharma daily new molecule launch and companies has to search for a unique Trade Name/Brand Name for it. One most common way to search unique brand name to search at IP INDIA website and know whether your brand name is resembling to any other or not. But there is lot of brands available which are not applied for brand name registration. That can’t be searched by it. So, there is maximum chances, any new chosen trade name could clash with any already existing trade name.
Manufacturing companies will ask for brand name affidavit because they want to confirm that you are not using any other company brand name. You Brand Name/Trade Name is unique and don’t resemble to any other Brand Name/trade Name available in Market. If in Future your Brand Name will clash with any other Company’s Brand Name, you will have total responsibility for it. Manufacturing company will not bear any responsibility and expenditure with regard to this brand name.
If one company is marketing by Name of Sovamax, you can’t choose any Trade Name/Brand Name Similar or resembling to it like Aovamax. It will be offense under Intellectual Property Act. You have to choose different Name like Kdakmax etc. For entering into contract manufacturing, you should set-up your new marketing company and have to choose brand names for it.













